For payers who may require a letter of Medical Necessity to process and cover RELISTOR claims.
File exceptions or prior authorization requests for Medicare Part D coverage.
Request a formulary exception to allow coverage for RELISTOR.
A guide to steps required, requested documentation, and correct forms.
Being proactive with PAs may lead to higher approval rates.1 Follow this helpful guide to request coverage of RELISTOR.
download ADDITIONAL resources
COMMON REASONS FOR PA DENIAL and how to avoid them
Double-check PA, fill in missing information, and resubmit.
Double-check PA, fill in missing information, and resubmit.
Confirm dosing
- RELISTOR tablets: RELISTOR 450 mg, three 150 mg tablets once daily (90 tablets/prescription)
- RELISTOR Subcutaneous Injection: RELISTOR 12 mg subcutaneously, once daily, as directed (28 prefilled syringes)
- Please refer to full Prescribing Information for dose adjustments in patients with renal or hepatic impairment
Confirm dosing
- RELISTOR tablets: RELISTOR 450 mg, three 150 mg tablets once daily (90 tablets/prescription)
- RELISTOR Subcutaneous Injection: RELISTOR 12 mg subcutaneously, once daily, as directed (28 prefilled syringes)
- Please refer to full Prescribing Information for dose adjustments in patients with renal or hepatic impairment
Confirm ICD-10-CM code and resubmit
The following ICD-10 codes can be considered for OIC:‡
- K59.03: Drug-induced constipation
‡The ICD-10-CM code and all other patient access-related information are provided for informational purposes only. It is the treating physician’s responsibility to determine the proper diagnosis, treatment, and applicable ICD-10-CM code. Salix Pharmaceuticals does not guarantee coverage or reimbursement for the product.
Confirm ICD-10-CM code and resubmit
The following ICD-10 codes can be considered for OIC:‡
- K59.03: Drug-induced constipation
‡The ICD-10-CM code and all other patient access-related information are provided for informational purposes only. It is the treating physician’s responsibility to determine the proper diagnosis, treatment, and applicable ICD-10-CM code. Salix Pharmaceuticals does not guarantee coverage or reimbursement for the product.
Include information on why RELISTOR is necessary and appropriate for the patient.
Ensure documentation of OTC laxative trial and failure or trial and failure of any other appropriate step therapies, if applicable.
Include information on why RELISTOR is necessary and appropriate for the patient.
Ensure documentation of OTC laxative trial and failure or trial and failure of any other appropriate step therapies, if applicable.
Confirm coverage; Medicare excludes certain drugs
- RELISTOR is not in a Medicare excluded category
Confirm coverage; Medicare excludes certain drugs
- RELISTOR is not in a Medicare excluded category
Be sure the below information is included and accurate on RELISTOR PAs
Age
18 years or older2
Suggested ICD-10-CM CODE FOR OIC*
K59.033: Drug-induced constipation
prEVIOUS THERAPIES TRIED AND FAILED
duration of therapy (eg, OTC laxatives and other relevant prescription therapies)
APPROVED DOSING FOR OIC
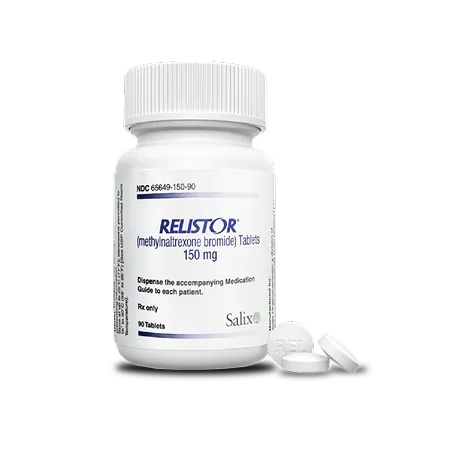

RELISTOR Tablets: 450 mg, three 150 mg tablets once daily (90 tablets/prescription)2
Please refer to full Prescribing Information for dose adjustments in patients with renal or hepatic impairment
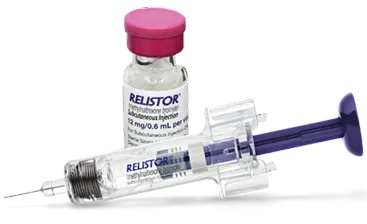

RELISTOR Subcutaneous Injection: 12 mg subcutaneously, once daily, as directed (28 prefilled syringes)2
ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; OIC, opioid-induced constipation.
*The ICD-10 code and all other patient access–related information are provided for informational purposes only. It is the treating physician’s responsibility to determine the proper diagnosis, treatment, and applicable ICD-10-CM code. Salix Pharmaceuticals does not guarantee coverage or reimbursement for the product.
Understand your local state laws regarding patient access
Access Step Therapy Laws by State
REFERENCES: 1. Data on File. PA Approval. Salix Pharmaceuticals, Bridgwater, NJ. 2. RELISTOR [prescribing information]. Bridgewater, NJ: Salix Pharmaceuticals. 3. ICD-10. Centers for Medicare & Medicaid Services: https://www.cms.gov/medicare/coding-billing/icd-10-codes.Updated February 26, 2025. Accessed March 20, 2025.

